INTRODUCTION
With the continued use and expanding applications of architectural concrete masonry, segmental retaining wall units, and concrete pavers, exposed concrete masonry is becoming common across the country. Although maintenance of a well designed and constructed masonry wall is minimal, inadvertent staining from oil, grease, or other foreign substances can destroy the appearance of an otherwise attractive unpainted masonry structure. This publication provides information on effective methods for removing some of the most common stains.
STAIN PREVENTION
Many stains can be prevented or minimized through proper design, construction, and maintenance procedures. For instance design details that prevent or reduce water intrusion reduce the chance that efflorescence will occur – see Maintenance of Concrete Masonry Walls, TEK 08-01A (ref. 1).
During construction of exposed concrete masonry, minimize mortar and grout smears on the face of the units. Mortar droppings which adhere to the exposed face of the units can be removed with a trowel or chisel after being allowed to harden. Any remaining mortar can then be removed with a stiff fiber brush. Also, the base of the wall should be protected from splashing mud and mortar droppings by spreading plastic sheets 3 to 4 feet on the ground and 2 to 3 feet up the wall. Covering the tops of unfinished walls at the end of the workday prevents rain from entering the wall and thus reduces the chance of efflorescence forming on the wall. Covers should be draped at least two feet down each side of the wall and a method provided to hold them in place. See Cleaning Concrete Masonry, TEK 08-04A (ref. 6) for more information on cleaning concrete masonry during construction and further information on cleaning concrete masonry.
PLANNING AND PRECAUTIONS
The cleaning procedure should be carefully planned. No attempt should be made to remove a stain until it is identified and its removal agent determined. If the staining substance cannot be identified, it is necessary to experiment with different methods on an inconspicuous area. The indiscriminate use of an inappropriate product or the improper application of a product may result in spreading the stain over a larger area or in causing a more unsightly, difficult to remove stain. Removing stains from concrete masonry sometimes can leave the treated area lighter in color than the surrounding area because surface dirt has been removed along with the stain or the surface has become slightly bleached. This is particularly true for buildings that are several years old. This may necessitate treating the entire wall. Materials such as glass, metal, wood or architectural concrete or concrete masonry adjacent to the area to be cleaned should be adequately protected since they may be damaged by contact with some stain removers or by physical cleaning methods.
Many chemicals can be applied to concrete masonry without appreciable injury to the surface, but strong acids or chemicals with a strong acid reaction definitely should be avoided. Even weak acids should be used only as a last resort as it dissolves the cement matrix of the masonry beginning at the surface. This leaves the face more porous so that it absorbs more water and exposes more aggregate thereby changing the color and texture of the masonry.
CLEANING METHODS
The methods of cleaning concrete masonry can generally be divided into three categories water cleaning, abrasive cleaning, and chemical cleaning (ref. 2).
Water Cleaning
Water cleaning includes the use of water soaking, steam cleaning and pressure washing. Cleaning of unpainted walls can usually be accomplished by scrubbing with water and a small amount of detergent. Clay or dirt first should be removed with a dry brush. Steel wire brushes should not be used because they can leave metal particles on the surface of the masonry that later may rust and stain the masonry. Nonmetal brushes such as stiff fiber or nylon are preferred. Soaking with water causes dirt deposits to swell, loosening their grip on the underlying masonry and then allowing them to be flushed away with water. Some efflorescence can be removed when it first appears by dry brushing followed by flushing with water. More extensive efflorescence may require brushing with acid see the section on chemical cleaning or Control and Removal of Efflorescence, TEK 08-03A (ref. 3).
Heated water is useful on greasy surfaces or during cold weather. However, warm water when used with alkaline chemicals, should not exceed 160° F (71° C). There is no significant advantage to using hot water with acid cleaners (ref. 2).
Steam cleaning virtually has been supplanted by improved and innovative pressure washing equipment. However, by supplementing heat to the soaking with water, the action of loosening and softening of dirt particles and grease is improved allowing them to be more easily rinsed away. The steam is normally generated in a flash boiler and directed toward the stain by means of a wand at a pressure of 10 to 80 psi depending on the equipment used. A drawback with steam cleaning is that is rather slow when compared to pressure washing. An advantage of steam cleaning is that it essentially leaves the concrete masonry surface intact.
High-pressure washing equipment can be extremely effective for restorative cleaning of older masonry; however, when improperly applied, it can cause severe damage. If pressure application of chemical cleaning agents is considered, the surfaces to be cleaned must be thoroughly prewetted, cleansing agents must be prediluted, and the application pressures should be kept to a minimum. High pressure washing, however, should not be mistaken as a total replacement for hand labor. The mild agitation created by brush application improves the overall cleaning results while enabling rinsing pressure to be kept to a minimum.

Abrasive Cleaning
The objective in abrasive cleaning is not to dissolve and wash away the stain, but to remove the outer portion of the masonry in which the stain is deposited. Included in this category are grinding wheels, sanding discs, sanding belts, and the more popular grit blasting. Silica sand in recent years has been replaced as the abrasive blasting material by other products such as crushed slag in the concern over health hazards posed by airborne silica dusts. Protective equipment and clothing must be used, including an approved respirator under a hood.
Care must be exercised when using abrasive cleaning techniques since over zealous applications can cause drastic changes to the appearance, durability, and water tightness of the masonry. To minimize this, softer, less damaging abrasives such as crushed cornhusks, walnut shells, glass beads, etc. can be used on more delicate surfaces. This process, sometimes called micro-peening, is slower and more costly and generally is not applicable to large scale cleaning operations.
Most of the dust that accompanies the dry process can be eliminated with wet abrasive cleaning by introducing water into the air-grit stream at the nozzle. However the smaller, harmful particles remain a health hazard so the same protective equipment and clothing are needed as for the dry process. The wet process requires the extra step of rinsing down the cleaned surface after blasting.
Needless to say, previously applied waterproofing agents are removed during the abrasive cleaning process. Therefore, they need to be reapplied after abrasive cleaning.
Chemical Cleaning
The popularity of chemical cleaning techniques has increased substantially in recent years. When used in conjunction with one of the water washing techniques previously described, chemical solvents dissolve staining materials and allow them to be washed away during the final rinsing process.
Many proprietary cleansing agents for removal of stains are available today. They are generally much safer for the user in that the chemicals are premixed so there virtually is no danger of mixing reactive chemicals and also for the masonry in that they are mixed in the proper proportions. Strict adherence to the manufacturer’s directions is still required, however, as improper use can still pose danger to both the user and the masonry. For the most part, products suitable for concrete are suitable for concrete masonry and can be found at most construction specialty and automotive supply centers and at hardware or paint stores.
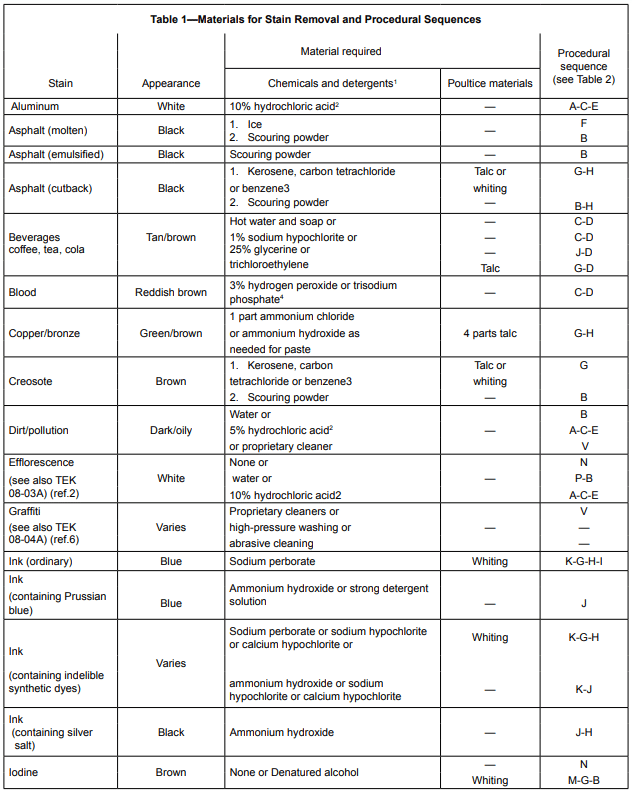
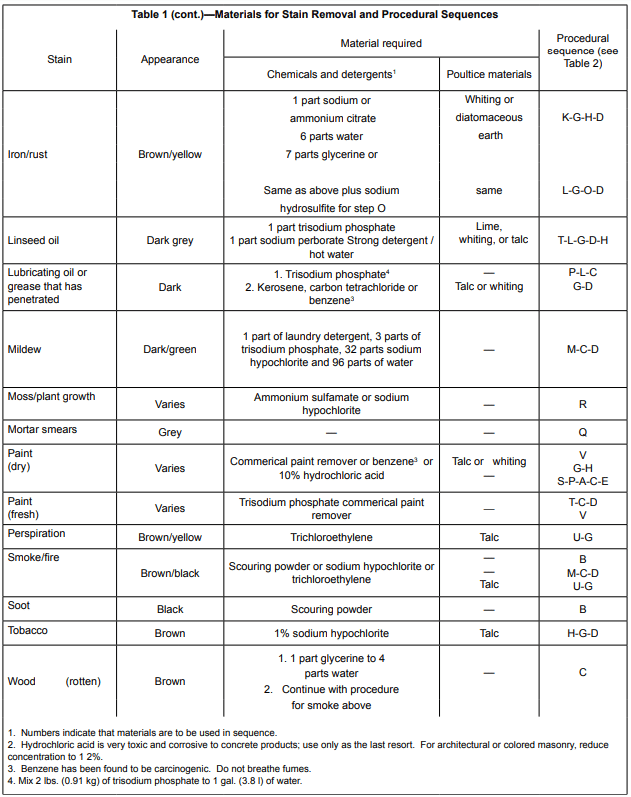
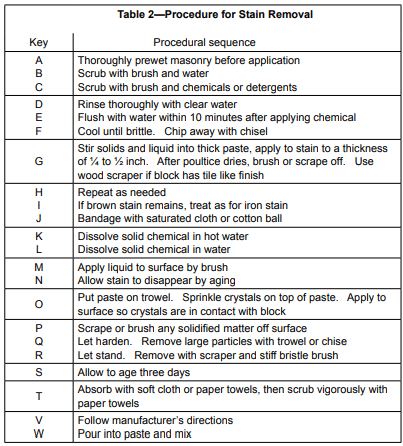
Tables 1 and 2 provide information covering the removal of many common materials that stain. Table 1 describes the chemicals, detergents, or poultice materials recommended for a particular stain. Table 1 also provides letter keys which indicate steps to be followed in the removal of the stain identified in Table 2.
A poultice is a paste made with a solvent or reagent and a finely powdered, absorbent, inert material used to keep stains from penetrating deeper or spreading. It also tends to pull the stain out of the pores. Enough of the solvent or reagent is added to a small quantity of the inert material to make a smooth paste. The paste is spread in a ¼ in. to ½ in. (6 to 13 mm) thick layer onto the stained area and allowed to dry. The solvent dissolves the staining substance and absorbs it into the poultice and is left as a loose, dried powdery residue that can be scraped or brushed off (ref.4). This process frequently takes several applications to remove the stain.
CHEMICAL SUBSTANCES
The following text provides general information on the chemicals and cleaning agents referenced in Table 1 (ref. 5). As with any chemical, refer to the chemical’s Material Safety Data Sheet and always follow label directions.
Ammonium Chloride (Other names: Amchlor, chloride of ammonia, darammon, salammonite)
Odorless white crystalline substance used in some agricultural processes. Available from chemical and dry-cleaning supply centers and hardware stores.
Hazards: Toxic and corrosive.
Ammonium Citrate (Other names: Citric acid, diammonium salt) White odorless substance in either granular or crystalline form. Found at supermarkets and hardware stores. Hazards: Corrosive and flammable.
Ammonium Hydroxide (Other names: Ammonia solution, ammonia water, household ammonia) A colorless liquid with a strong irritating odor. Found at most supermarkets and hardware stores. Hazards: Toxic.
Ammonium Sulfamate (Other names: Amicide, ammonium amidosulphate) A white crystalline substance commonly used as a weed killer. Found at chemical and garden supply centers. Hazards: None.
Benzene (Other names: Benzol, benzole, coal naptha) An excellent solvent and colorless liquid with characteristic odor and burning taste. Found at automotive, chemical and dry cleaning supply centers and hardware and paint stores. Hazards: Violently flammable and carcinogenic
Calcium Hypochlorite (Other names: B-K Powder, losantin, pool chlorine) White in powder, granule, or pellet form used to kill algae, fungus, and bacteria. Found in pool chemical and garden supply centers. Hazards: Corrosive to flesh and flammable when in contact with organic solvents.
Carbon Tetrachloride (Other names: Perchloromethane, tetrachloromethane) A nonflammable, clear, poisonous liquid used in fire extinguishers and as a solvent. Available at chemical, dry cleaning, and pharmaceutical supply centers, and paint stores. Hazards: Toxic.
Glycerine (Other names: Glycerol, glycyl alcohol) An odorless, colorless, syrupy liquid prepared by the hydrolysis of fats and oils. Found at chemical, pharmaceutical, photographic, and printer supply centers. Hazards: Flammable.
Denatured Alcohol (Other names: Methylated Spirit) Found at pharmaceutical and printer supply centers and hardware stores. Hazards: Toxic and flammable.
Hydrochloric Acid (Other names: Muriatic acid) A strong, highly corrosive acid commonly used for cleaning metals and balancing the pH of swimming pools. It can be found at swimming pool supply centers, chemical supply centers and hardware stores. Hazards: Toxic, very corrosive to flesh and concrete materials. Reacts vigorously with ammonia and detergents containing ammonia. Use extreme caution when handling and applying. Never use full strength. Dilute by adding acid to water, never water to acid. Rinse thoroughly within 10 minutes after applying.
Hydrogen Peroxide (Peroxide of hydrogen) A colorless, syrupy liquid used as a bleaching and disinfectant in low concentrations and as a rocket fuel in higher concentrations. Available at chemical supply centers, drug stores, supermarkets, and hardware stores. Hazards: None in the normal 3% solution. Toxic, corrosive to flesh and flammable in higher concentrations.
Sodium Citrate (Other names: Citrate of soda, trisodium citrate) White odorless substance in crystalline, granular, or powder form. Commonly used as a neutralizing buffer in chemical research. Available from chemical supply centers and drug stores. Hazards: None.
Sodium Hydrosulfite (Other names: Hydrolin) White powder with little odor. Commonly used in industrial cleaners. Found at chemical supply centers. Hazards: Very toxic when in contact with moisture.
Sodium Hypochlorite (Other names: Clorox, hypochlorous acid, household bleach) Faint yellow to clear liquid with chlorine smell. Available at supermarkets. Hazards: Corrosive to flesh.
Sodium Perborate (Other names: Perboric acid, perborax, sodium salt) White, odorless, crystalline powder commonly found in “allinone” laundry detergents and some dishwashing powders. Available at chemical and pharmaceutical supply centers and supermarkets. Hazards: Toxic and flammable when in contact with organic solvents.
Trichloroethylene (Other names: TCE, ethynyl trichloride) Colorless liquid with chloroform smell found in common cleaning solvents. Available at automotive, chemical, dry cleaning, paint, photographic, and printer’s supply centers. Hazards: Highly toxic and can react with strong alkalies in fresh mortar or concrete to form dangerous gases.
Trisodium Phosphate (Other names: Sodium orthophosphate, TSP, phosphate of soda) A crystalline, white, odorless compound found in household cleaning detergents such as “Spic and Span”. Available at supermarkets and hardware stores. Hazards: Corrosive to flesh
MATERIALS FOR POULTICES
The main properties desired in the powdered materials used to make poultices are: 1) grains sufficiently fine so the paste will hold plenty of liquid; 2) enough range in particle size so they will make a smooth, readily moldable paste; and 3) chemical inertness to the chemicals with which the powdered material is used. The last precludes using portland cement in combination with water, although it can be used with organic liquids. For the same reason, if acids are to be used, the paste must not be made with whiting (calcium carbonate), ground limestone, hydrated lime, or portland cement. Otherwise, the finely divided materials are more or less interchangeable.
Diatomaceous Earth (Other name: Diatomite, filter media, fuller’s earth) Available at swimming pool supply centers.
Lime (Other names: Calcium hydroxide, caustic lime, mason’s lime, quicklime) Available at building material supply centers and nurseries.
Portland Cement (Other names: Cement) Found at building material supply centers and ready mixed concrete plants.
Talc (Other names: Talcum powder) A very soft mineral that is a basic silicate of magnesium, has a soapy feel, usually white in color, and is used especially in making talcum powder. Available at supermarkets and drug stores.
Whiting (Other names: Calcium carbonate, baking powder) Found in nature as calcite and aragonite and in plant ashes, bones and shells. Available at supermarkets and nurseries.
REFERENCES
- Maintenance of Concrete Masonry Walls, TEK 08-01A, Concrete Masonry & Hardscapes Association, 2004
- Grimm, Clayford T., Cleaning Masonry A Review of the Literature, Construction Research Center, University of Texas at Arlington, November 1988.
- Control and Removal of Efflorescence, TEK 08-03A. Concrete Masonry & Hardscapes Association, 2003.
- Removing Stains and Cleaning Concrete Surfaces, Portland Cement Association, 1988.
- Removing Stains from Concrete, Concrete Construction Publications, Inc., May 1987.
- Cleaning Concrete Masonry, TEK 08-04A, Concrete Masonry & Hardscapes Association, 2005.
